If Avogadro number `N_(A)` is changed from `6.022xx10^(23) mol^(-1)` to 6`.022xx10^(23) mol^(-1)`, - YouTube

Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com

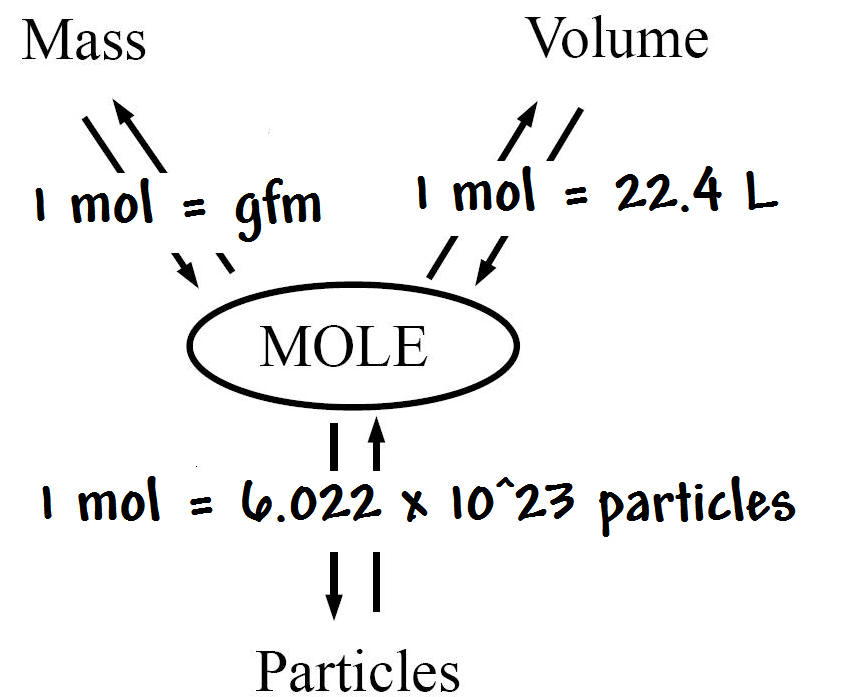
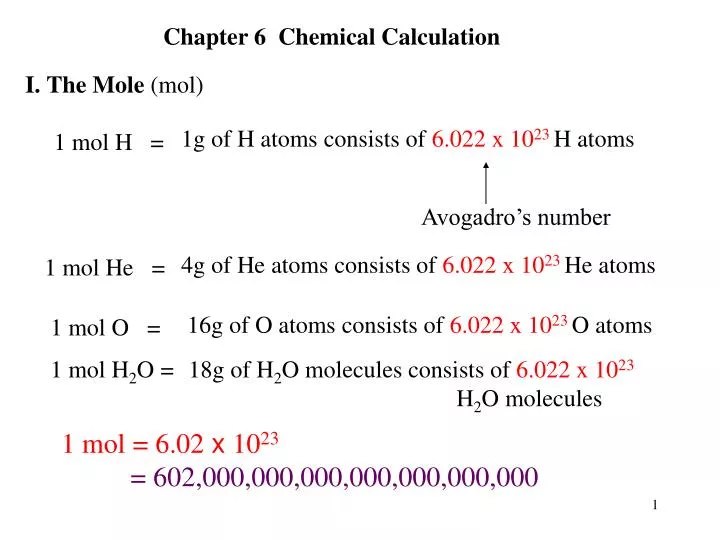
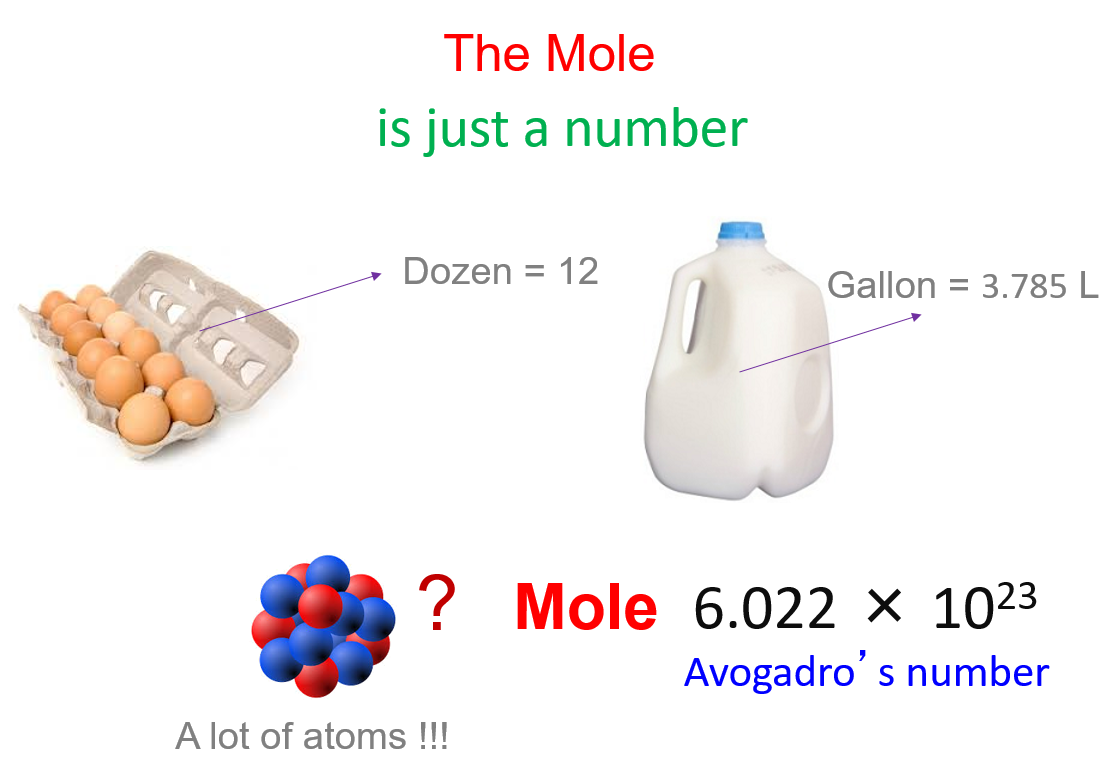
Oh My!!. Mole (mol) can be defined as the number equal to the number of carbon atoms in grams of carbon (in an chemical equation it is the coefficients. - ppt download

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com
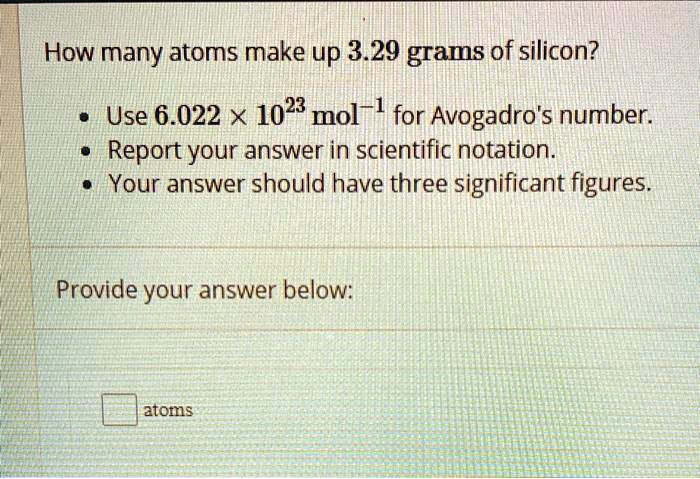
SOLVED: How many atoms make up 3.29 grams of silicon? Use 6.022 X 1023 mol-l for Avogadro's number: Report your answer in scientific notation. Your answer should have three significant figures Provide

1 mole = 6 022 x 10^23 If there is 1 mole of H2 we have multiply the Avogadro no - Science - Atoms and Molecules - 15776529 | Meritnation.com